Entitled “Wearable Disposable Electrotherapy,” the CCNY team’s work appears in the prestigious journal Nature Communications. “It’s a novel platform for medicine. Single-use millimeter-thick adhesive patches, each tuned to deliver a specific therapy. Applications include enhancing the skins healing processes, applying energy to treat brain disorders, and delivering drugs through the skin.”, said Bikson, who leads the Neural Engineering Group in the Grove School.
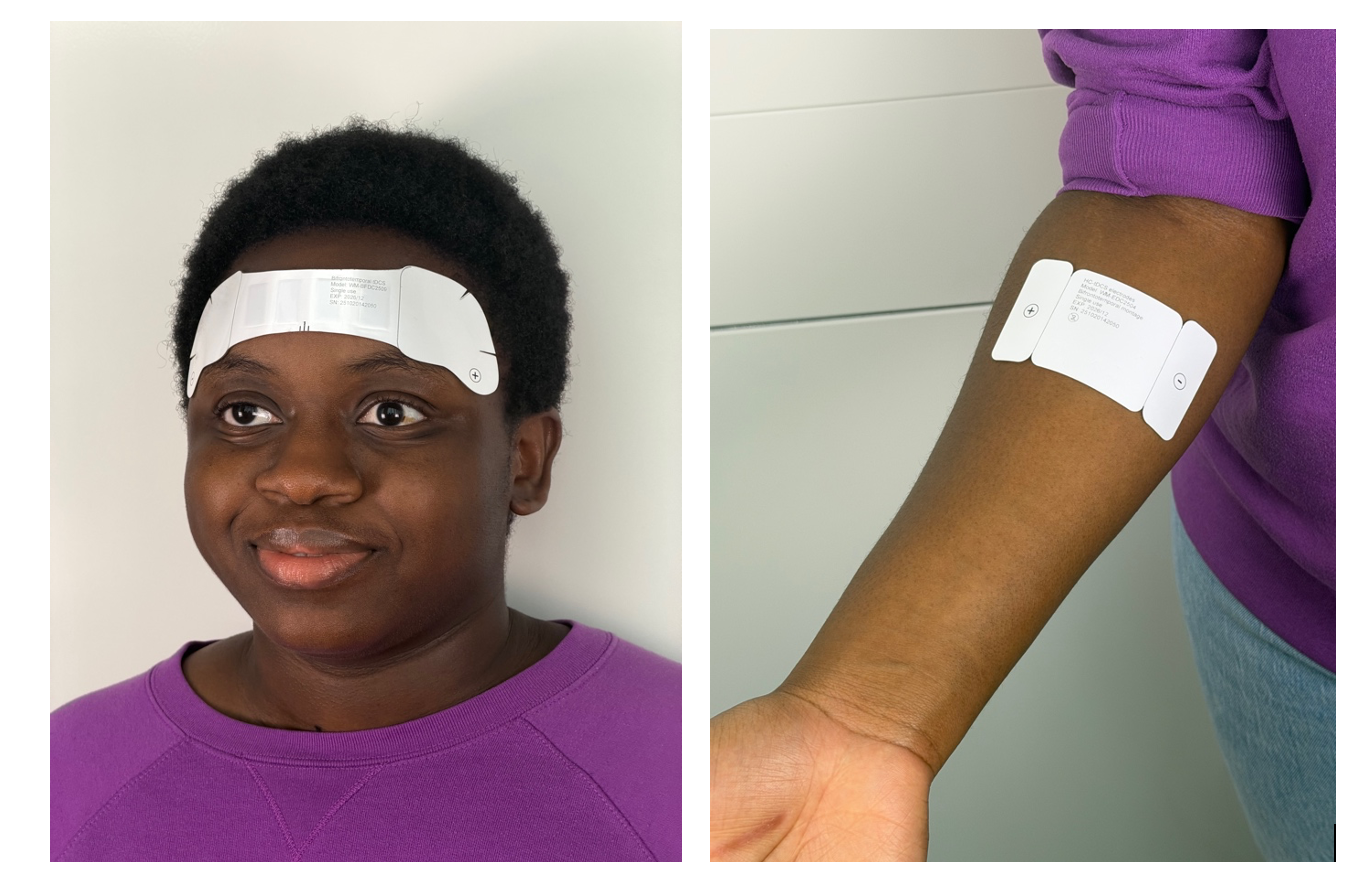
The disposable single-use devices, which is as discreet as adhesive bandages, is activated simply by placement on the body. The device senses the body and, over a few minutes or an hour, delivers a single therapy dose. Then device can then be removed and thrown away. Bikson commented “We call is wearable medicine.”
What makes Wearable Disposable Electrotherapy a technological breakthrough is that each patch is a thin electronic device able to deliver a therapeutic dose, but there are no packaged batteries or electrical components. Couzis explains “Since each patch is single use and disposable, we needed environmentally benign materials - so, no electronics. Wearable Disposable Electrotherapy is the first electronics-free device that can sense and change the body. It took over six years and multiple innovation in chemical, electrical, and biomedical engineering achieve this product.”
The prescribed therapy dose is regulated by a flexible architecture consisting of dozens of printed chemicals components- together they form a thin 3D electrochemical. “Without using any electrical components, we created a device that self-powers and regulates therapy out of its electrochemical network. For each application, such as wound healing, or electrical therapy or a drug-delivery patch, a unique electrochemical structure is created.” But using only scalable additive printing technology and abundant materials, the cost of each device is reduced to pennies.
The use of the device determines its shape and function. For wound healing and skin enhancement applications, Wearable Disposable Electrotherapy are made like adhesive bandages, but the added benefit of bioelectric healing. For uses such as migraine, depression, or dementia a path across the forehead delivery therapeutic electricity to the brain. For drug delivery, such a pain medication or even vaccines, the drug is also built into the device which delivers it through the skin when the patch is applied.
Wearable Disposable Electrotherapy has already been featured in conferences including the International Brain Stimulation Conference and the Neuroscience of the Everyday World where the physician and scientists audiences hailed it as a “transformative technology” and a “new category of medicine”.
Both Dr. Couzis and Dr. Bikson has previously launch successful City College of New York spinoffs including having started Urban Electrical Power and Soterix Medical, respectively.
“We have produced prototypes for each application and proven they deliver the prescribed therapy. We are now planning clinical trials. For each use case we are working with leading medical centers to test therapy efficacy,” said Bikson.
So, the next time instead of a pill or needle you’re offered an adhesive patch, you’ll know where it was invented.
Addition Q&A with the inventors:
1. What inspired the development of the "Wearable Disposable Electrotherapy" concept, and how did your team first envision electronic-free wearable medicine?
There are many medical conditions for which drug therapy is either insufficiently effective or associated with unwanted side effects. Electrotherapy is a promising alternative, but current devices are often bulky and difficult for patients to use. Wearable Disposable Electrotherapy brings together the therapeutic advantages of electrotherapy with the simplicity and usability of pharmacotherapy.
2. The patches are described as single-use, battery-free, and electronics-free. Could you explain the mechanism by which they generate and deliver therapeutic energy without traditional power components?
Conventional electrotherapy devices rely on packaged batteries and electronic circuits to control stimulation. Because our goal was to create a single-use device that is inexpensive and environmentally benign, we developed an electronics-free electrotherapy delivery system. Each millimeter-thin patch contains a network of electrochemical components that become activated upon skin contact. This novel electrochemical architecture self-regulates to deliver one controlled therapeutic dose. By using only abundant materials and scalable printing-based manufacturing, we keep both complexity and cost to a minimum.
3. Your paper highlights applications ranging from wound healing to neurological therapy and drug delivery. Which of these areas do you see as the most promising for near-term clinical translation?
Wearable Disposable Electrotherapy is a platform that can be tuned for many therapeutic applications. We are currently focused on three use cases for near-term clinical translation.
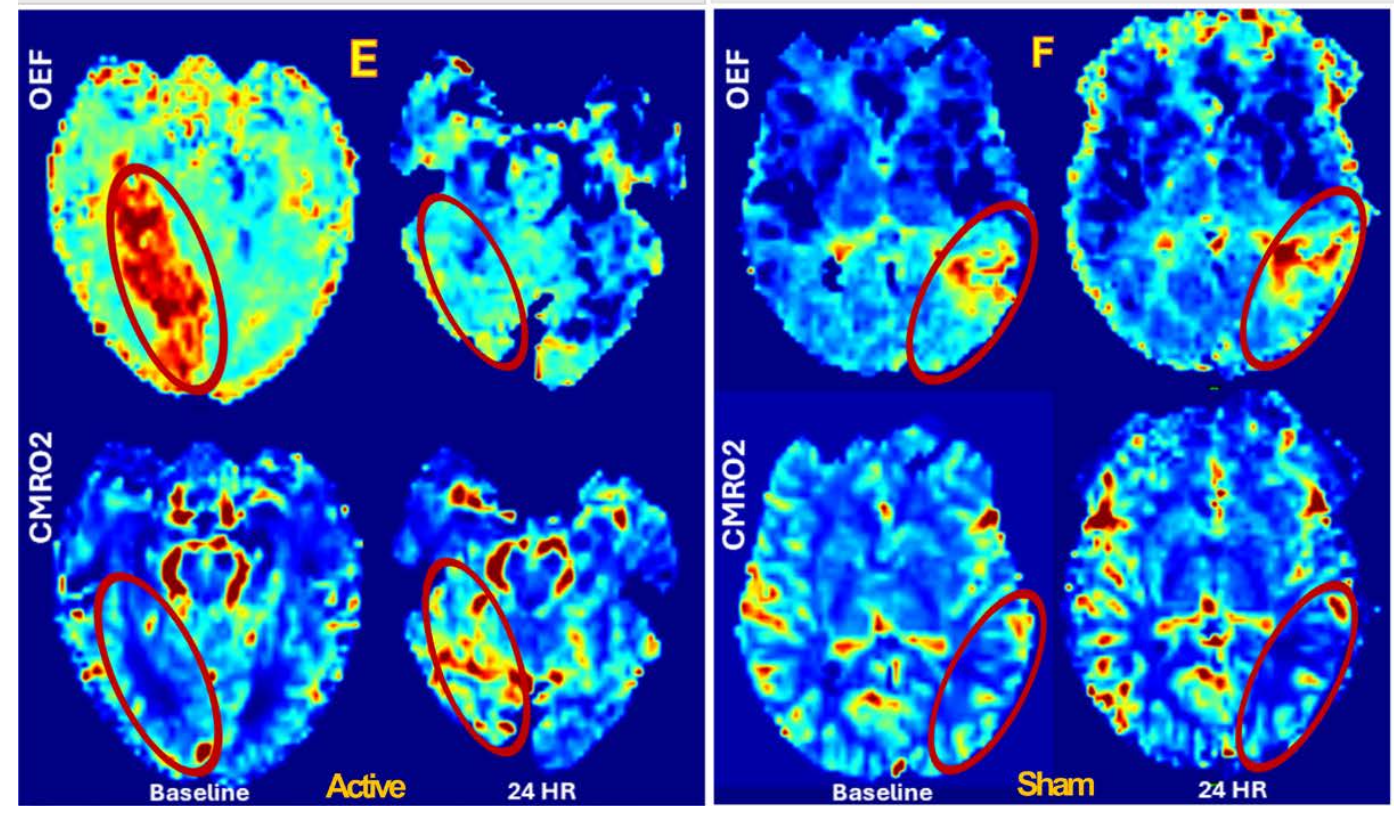
First, we demonstrated that Wearable Disposable Electrotherapy bandages can accelerate wound healing, reduce infection, and minimize complications; clinical trials will begin with post-surgical wound care.
Second, forehead-applied patches will be tested for depression and migraine therapy—essentially bringing brain stimulation into a simple single-use format.
Third, we are developing versions designed for delivering drugs through the skin without needles.
4. Environmental sustainability is an important consideration for single-use devices. How did your team ensure the materials used in these patches are safe and eco-friendly?
Environmental sustainability was a core design priority from the outset. We rebuilt the electrotherapy platform around simple, inert, mineral-based materials. The patches use only microgram quantities of naturally occurring, environmentally safe minerals arranged in thin printed layers to form a functional electrotherapy system. Because the device contains no electronics and is manufactured using low-impact processes, its overall footprint is dramatically lower than that of conventional electrotherapy devices, which rely on disposable electrodes plus electronic housings, batteries, and resource-intensive fabrication. Our approach replaces all of this with a clean, minimal, eco-conscious alternative.
5. What challenges did you face in designing a device that successfully integrates chemical, electrical, and biomedical principles while remaining simple enough for disposable, everyday use?
Developing this platform required nearly a decade of iterative design. The process was inherently recursive—advances in one component often required revisiting and improving others. Integrating chemistry, electrochemistry, material science, and bioengineering while keeping the device simple, manufacturable, and comfortable for patients was deeply complex. We also had to consider patient experience, real-world therapy distribution, and cost constraints simultaneously, making this one of the most challenging but rewarding engineering problems we have tackled.
6. Looking ahead, what are the next steps for this technology—are there plans for clinical trials, partnerships with medical device companies, or commercial development?
Commercialization will proceed through our new venture, Wearable Medicine. Clinical trials are underway to validate the technology for specific therapeutic indications. We are actively pursuing partnerships with investors and medical device companies that recognize the transformative potential of Wearable Disposable Electrotherapy—the first medical technology to unite the strengths of pharmacotherapy with those of electrotherapy.