The National Institutes of Health NATIONAL INSTITUTE OF GENERAL MEDICAL SCIENCES awarded “BRIDGES TO THE BACCALAUREATE RESEARCH TRAINING PROGRAM AT LAGUARDIA COMMUNITY COLLEGE” to a team including LAGUARDIA COMMUNITY COLLEGE (PI Hendrick Delcham) and THE CITY COLLEGE OF NEW YORK (M-PI Marom Bikson).
LaGuardia Community College’s “Bridges to the Baccalaureate Program” provides mentored research (including at The City College of New York) experiences year round to qualified minority, economically disadvantaged or disabled students. Beyond the research experience, the program features instructional workshops on quantitative literacy (Biostatistics), oral presentation, research paper critiques, bio- instrumentations, research design, data science, mentoring, leadership and management skills, monthly research student seminars, tutoring, transfer and transfer counseling, student presentations at local and national conferences.
LaGuardia Community College’s “Bridges to the Baccalaureate Research Training Program” has demonstrated high graduation and high transfer rates for our students, conclusively demonstrating that a community college can take the lead in administering a successful Bridges program. Our program has formed a consortium LaGuardia Community College’s and three exceptional four-year colleges—the City College of New York, Hunter College, and Queens College—to provide challenging research experiences in the biomedical and behavioral sciences for our underrepresented college students: women, minorities, the disabled, and those from economically disadvantaged backgrounds. LaGuardia proposes to place 10 students in hands-on, mentored research experiences each year of the grant period. These students will choose from a list of research projects and will be engaged in preliminary, preparatory research at LaGuardia, under the tutelage of the LaGuardia Faculty Research Mentors. This experience gained will then be utilized during the summer, as the Bridges students become involved in more intensive research at our three linking colleges, Brookhaven National Laboratories, and SUNY downstate Medical Center. The Bridges program also features a number of activities designed to support the students: monthly research student seminars, tutoring, transfer counseling, opportunities to present their research results at local and national conferences, instruction in the Responsible Conduct of Research, Rigor and Reproducibility, instructional workshops on bio-statistics, leadership and self-management skills, bioinstrumentation, research paper critique, library research, research design, data science, introduction to Python, and poster presentation and the use of ePortfolios. The ePortfolio will be used by Bridges students to collect their academic work, progress report and to reflect on their learning and career goals. The program will also offer LaGuardia faculty the opportunity to participate in effective mentoring workshop offered at the university of Wisconsin and Bridges students will also enroll in the National Research Mentoring Network (NRMN). The monthly research seminars are notable in that they feature progress reports and formal final reports by the students themselves, presentations by CUNY faculty and outside speakers, information from the program’s transfer counselor, a session on developing and delivering professional presentations, and an Alumni Homecoming Day where Bridges alumni return to share their successes and research with current Bridges students. Bridges students will also use an adapted version of myIDP (Individual Development Plan) to explore careers in biomedical, sciences, and bioengineering.
Design and Rationale of the PACt-MD Randomized Clinical Trial: Prevention of Alzheimer's dementia with Cognitive remediation plus transcranial direct current stimulation in Mild cognitive impairment and Depression
Rajji TK, Bowie CR, Herrmann N, Pollock BG, Bikson M, Blumberger DM, Butters MA, Daskalakis ZJ, Fischer CE, Flint AJ, Golas AC, Graff-Guerrero A, Kumar S, Lourenco L, Mah L, Ovaysikia S, Thorpe KE, Voineskos AN, Mulsant BH; PACt-MD Study Group. .
J Alzheimers Dis . 2020;76(2):733-751. doi: 10.3233/JAD-200141. PDF
Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation (tDCS) in neurological and psychiatric disorders.
Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, Brunelin J, Nakamura-Palacios EM, Marangolo P, Venkatasubramanian G, San-Juan S, Caumo W, Bikson M, Brunoni AR, Neuromodulation Center Working Group.
Int J Neuropsychopharmacol . 2020 Jul 26;pyaa051. doi: 10.1093/ijnp/pyaa051.
In press PDF
Niranjan Khadka, Marom Bikson. Neurovascular-modulation. bioRxiv 13046435 2020. DOI: https://doi.org/10.1101/2020.07.21.214494
Download PDF published in bioRxiv — DOI
Abstract
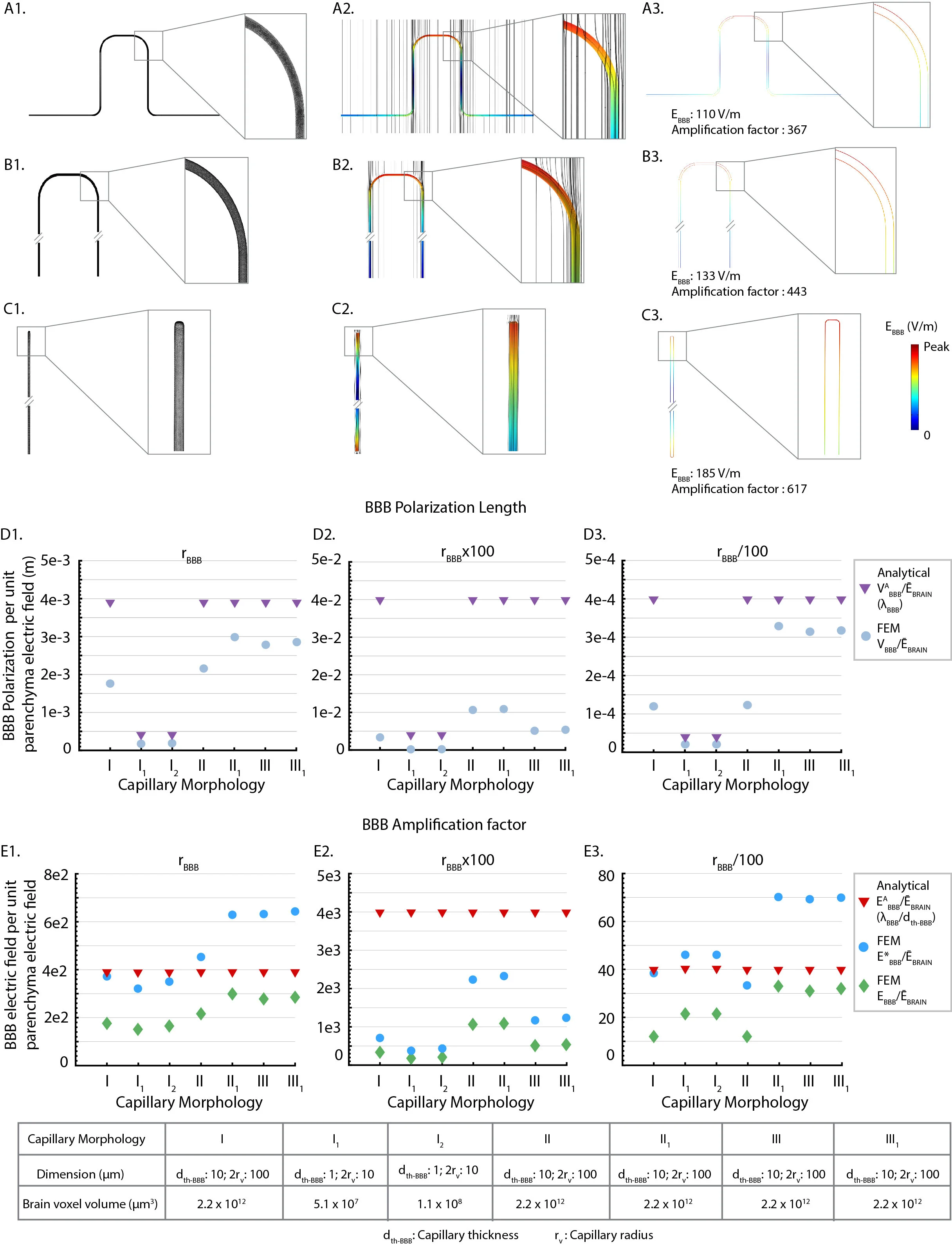
Neurovascular-modulation is based on two principles that derive directly from brain vascular ultra-structure, namely an exceptionally dense capillary bed (BBB length density: 972 mm/mm3) and a blood-brain-barrier (BBB) resistivity (ρ ~ 1x105 Ω.m) much higher than brain parenchyma/interstitial space (ρ ~ 4 Ω.m) or blood (ρ ~ 1 Ω.m).Principle 1: Electrical current crosses between the brain parenchyma (interstitial space) and vasculature, producing BBB electric fields (EBBB) that are > 400x of the parenchyma electric field (ĒBRAIN), which in turn modulates transport across the BBB. Specifically, for a BBB space constant (λBBB) and wall thickness (dth-BBB): analytical solution for maximum BBB electric field (EABBB) is given as:(ĒBRAIN x λBBB) / dth-BBB. Direct vascular stimulation suggests novel therapeutic strategies such as boosting metabolic capacity or interstitial fluid clearance. Boosting metabolic capacity impacts all forms of neuromodulation, including those applying intensive stimulation or driving neuroplasticity. Boosting interstitial fluid clearance has broad implications as a treatment for neurodegenerative disease including Alzheimer's disease.Principle 2: Electrical current in the brain parenchyma is distorted around brain vasculature, amplifying neuronal polarization. Specifically, vascular ultra-structure produces ~50% modulation of the average ĒBRAIN over the ~40 μm inter-capillary distance. The divergence of EBRAIN (activating function) is thus ~100 kV/m2 per unit average ĒBRAIN. This impacts all forms of neuromodulation, including Deep Brain Stimulation (DBS), Spinal Cord Stimulation (SCS), Transcranial Magnetic Stimulation (TMS), Electroconvulsive Therapy (ECT), and transcranial electrical stimulation (tES) techniques such a transcranial Direct Current Stimulation (tDCS). Specifically, whereas spatial profile of EBRAIN along neurons is traditionally assumed to depend on macroscopic anatomy, it instead depends on local vascular ultra-structure.
Callesen H, Habelt B, Wieske F, Jackson M, Khadka N, Mattei D, Bernhardt N, Heinz A, Liebetanz D, Bikson M, Padberg F, Hadar R, Nitsche MA, Winter C
Download: PDF published in Nature Translational Psychiatry – DOI
Download Supplementary figures
Abstract
Involuntary movements as seen in repetitive disorders such as Tourette Syndrome (TS) results from cortical hyperexcitability that arise due to striato-thalamo-cortical circuit (STC) imbalance. Transcranial direct current stimulation (tDCS) is a stimulation procedure that changes cortical excitability, yet its relevance in repetitive disorders such as TS remains largely unexplored. Here, we employed the dopamine transporter-overexpressing (DAT-tg) rat model to investigate behavioral and neurobiological effects of frontal tDCS. The outcome of tDCS was pathology dependent, as anodal tDCS decreased repetitive behavior in the DAT-tg rats yet increased it in wild-type (wt) rats. Extensive deep brain stimulation (DBS) application and computational modeling assigned the response in DAT-tg rats to the sensorimotor pathway. Neurobiological assessment revealed cortical activity changes and increase in striatal inhibitory properties in the DAT-tg rats. Our findings show that tDCS reduces repetitive behavior in the DAT-tg rat through modulation of the sensorimotor STC circuit. This sets the stage for further investigating the usage of tDCS in repetitive disorders such as TS.
The Realistic Anatomically Detailed Open-source Spinal Cord Stimulation Model (RADO-SCS) is the most anatomically detailed and open-source spinal cord model for simulating all forms of Spinal Cord Stimulation (SCS), DRG stimulation, other forms of spinal cord modulation such as transpinal Direct Current Stimulation (tsDCS), and other forms of spinal modeling such as biomechanical. RADO-SCS is an open source spinal cord stimulation model designed in Solidworks 2016. Model includes detailed structures of the lower thoracic vertebrae (T10-T12) and the spinal column with an emphasis on spinal tissues, nerves, and vasculature. Layers of meninges protecting the gray and white matter such as epidural space, subdural space, arachnoid matter, CSF, and pia matter are designed in detail. Lissauer’s tract and rootlets carrying nerve fibers away from the spinal cord are also included in anatomical detail. STL and Solidworks files for this open source model, as well as any questions on use, can be requested at https://www.neuralengr.org/spinal-cor...
This work has been conducted in collaboration between Dr. Marom Bikson's and Scott Lempka’s research groups. This model of this video was published as and can be cited as: Khadka, N., Liu, X., Zander, H., Swami, J., Rogers, E., Lempka, S., Bikson, M., 2020. Realistic anatomically detailed open-source spinal cord stimulation (RADO-SCS) model. J. Neural Eng. https://doi.org/10.1088/1741-2552/ab8344
This talk given at BioKorea 2020 explains "High-Definition Transcranial Direct Current Stimulation (HD-tDCS) : Low-power, Targeted, Non-invasive Electroceuticals for CNS diseases".
HD-tDCS is special among neuromodulation approaches in that it 1) can be delivered with a battery powered device, 2) it is non-invasive and very well tolerated, can be fully wearable, 3) can be targeted to anatomical regions including using individual MRI or EEG, 4) and can be functionally targeted since it is sub-threshold. No other brain stimulation technique combines all these features, and this talk explains each one in turn.
Watch the talk here
Download the talk PDF
All references (and more) can be found on the lab publication page here
$3 million NIH grant boosts CCNY minority PhD output
In a massive boost to its development of minority PhD students in biomedical disciplines, The City College of New York is the recipient of a new five-year $2,966,693 training grant from the National Institutes of Health (NIH). Prof. Marom Bikson and Prof. Lucas Parra are among 30 participating faculty,
Full press release here
A prospective trial of intraoperative tissue oxygenation measurement and its association with anastomotic leak rate after Ivor Lewis esophagectomy. J Thorac Dis 2020;12(4):1449-1459
Adusumilli PS, Bikson M, Rizk NP, Rusch VW, Hristov B, Grosser R, Tan KS, ISarkaria IS, Huang J, Molena D, Jones DR, Bains MS.
http://dx.doi.org/10.21037/jtd.2020.02.58
Download the PDF
Brainbox Initiative Webinar: The Targeting Limits of Transcranial Electrical Stimulation. Marom Bikson of the The City College of New York
Hear the talk here
Download PDF slide
Some additional Q&A posted here
This free, interactive session will equip delegates with a knowledge of: Modes of transcranial electrical stimulation including conventional tDCS and High-Definition tDCS. Insights on the mechanisms of tDCS that integrate results from advanced current flow and animal models. Using EEG to guide stimulation (reciprocity). New concepts in non-invasive sub-gyri targeting. Functional targeting and Hebbian neuromodulation. The uses and pitfalls of Anode/Cathode based intervention design. Automated tools for individuated modeling. Biophysical insights into Temporal interference stimulation. This webinar will take place at 14:00 BST on May 18, 2020 and will last for approximately 1 hour with time for questions.
New publication:
Guidelines for TMS/tES Clinical Services and Research through the COVID-19 Pandemic
Read it : online
Bikson M, Hanlon CH, Woods AJ, Gillick BT, Charvet L, Lamm C, Madeo G, Holczer A, Almeida J, Antal A, Ay MR, Baeken C, Blumberger DM, Campanella S, Camprodon J, Christiansen L, Colleen L, Crinion J, Fitzgerald P, Gallimberti L, Ghobadi-Azbari P, Ghodratitoostani I, Grabner R, Hartwigsen G, Hirata A, Kirton A, Knotkova H, Krupitsky E, Marangolo M, Nakamura-Palacios EM, Potok W, Praharaj SK, Ruff CC, Schlaug G, Siebner HR, Stagg CJ, Thielscher A, Wenderoth N, Yuan T, Zhang X, Ekhtiari H. . 2020
Dr. Marom Bikson leads with Dr. Hamed Ekhtiari and international team on experts.
We developed a framework for balancing the importance of NIBS operations with safety considerations, which facilitates the re-establishment of access to NIBS clinical services and research operations during COVID-19.
The present consensus paper provides guidelines and good practices for managing and reopening NIBS clinics and laboratories through the immediate and ongoing stages of COVID-19.
The proposed robust and structured strategy aims to address the current and anticipated future challenges while maintaining scientific rigor and managing risk.
Published in Brain Stimulation:
Transcranial Electrical Stimulation Motor Threshold Can Estimate Individualized tDCS Dosage from Reverse-Calculation Electric-Field Modeling
Kevin A. Caulfield, Bashar W. Badran , William H. DeVries, Philipp M. Summers, Emma Kofmehl, Xingbao Li, Jeffrey J. Borckardt, Marom Bikso,n Mark S. George
Free online
Reverse-calculation electric-field modeling can estimate individualized tDCS doses.
Individualized tDCS doses widely vary (range: 3.75 to 9.74mA to produce 1V/m).
•DCS applied at a uniform 1-2mA dose may underdose some individuals.
Transcranial electrical stimulation (TES) motor thresholds (MTs) correlate with reverse-calculation tDCS doses (R2 = 0.45, p < 0.001).
TES MT or reverse-calculation modeling could become methods to individually dose tDCS and warrant further investigation.
Prof. Marom Bikson among the scientists featured in Freethink article “Treating Depression at Home with a tDCS Headset”
Read it here
New publication: Brain Stimulation Journal VOLUME 13, ISSUE 3, P686-693, MAY 01, 2020
Supervised transcranial direct current stimulation (tDCS) at home: A guide for clinical research and practice Leigh E. Charvet. Michael T. Shaw, Marom Bikson, Adam J. Woods Helena Knotkova
Background: Transcranial direct current stimulation (tDCS) is a method of noninvasive neuromodulation and potential therapeutic tool to improve functioning and relieve symptoms across a range of central and peripheral nervous system conditions. Evidence suggests that the effects of tDCS are cumulative with consecutive daily applications needed to achieve clinically meaningful effects. Therefore, there is growing interest in delivering tDCS away from the clinic or research facility, usually at home. Objective: To provide a comprehensive guide to operationalize safe and responsible use of tDCS in home settings for both investigative and clinical use. Methods: Providing treatment at home can improve access and compliance by decreasing the burden of time and travel for patients and their caregivers, as well as to reach those in remote locations and/or living with more advanced disabilities. Results: To date, methodological approaches for at-home tDCS delivery have varied. After implementing the first basic guidelines for at-home tDCS in clinical trials, this work describes a comprehensive guide for facilitating safe and responsible use of tDCS in home settings enabling access for repeated administration over time. Conclusion: These guidelines provide a reference and standard for practice when employing the use of tDCS outside of the clinic setting.
Niranjan Khadka, Xijie Liu, Hans Zander, Jaiti Swami, Evan Rogers, Scott F Lempka, Marom Bikson. Realistic anatomically detailed open-source spinal cord stimulation (RADO-SCS) model. Journal of Neural Engineering 2020. DOI: 10.1088/1741-2552/ab8344
Download PDF published in Journal of Neural Enginnering — DOI
Abstract
Objective:
Computational current flow models of spinal cord stimulation (SCS) are widely used in device development, clinical trial design, and patient programming. Proprietary models of varied sophistication have been developed. An open-source model with state-of-the-art precision would serve as a standard for SCS simulation.
Approach:
We developed a sophisticated SCS modeling platform, named Realistic Anatomically Detailed Open-Source Spinal Cord Stimulation (RADO-SCS) model. This platform consists of realistic and detailed spinal cord and ancillary tissues anatomy derived based on prior imaging and cadaveric studies. In our finite element model of the T9-T11 spine levels, we represented the following tissues: vertebrae, intervertebral disc, epidural space, epidural space vasculature, dura mater, dural sac, intraforaminal tissue, cerebrospinal fluid (CSF), whitematter, spinal cord vasculature, Lissauer’s tract, gray matter, dorsal and ventral roots and rootlets, dorsal root ganglion (DRG), sympathetic chain (trunk and ganglion), thoracic aorta and its branching, peripheral vasculature, and soft tissues (thorax). As an exemplary application to illustrate the model workflow, we simulated a bipolar SCS montage and calculated the corresponding activation thresholds for individual axons populating the spinal cord.
Main results:
RADO-SCS provides state-of-the-art precision across 19 tissue compartments. The resulting model calculations of the electric fields generated in the white-matter and gray matter, and the axonal activation thresholds are broadly consistent with prior simulations.
Significance:
The RADO-SCS can be used to simulate any SCS approach with both unprecedented resolution (precision) and transparency (reproducibility). Freely-available online, the RADO-SCS will be updated continuously with version control.
Transcranial Electrical and Magnetic Stimulation (tES and TMS) for Addiction Medicine: A consensus paper on the present state of the science and the road ahead. https://doi.org/10.1016/j.neubiorev.2019.06.007. PDF
Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, Castelo-Branco L, Challet-Bouju G, Clark VP, Claus E, Dannon PN, Del Felice A, den Uyl T, Diana M, di Giannantonio M, Fedota JR, Fitzgerald P, Gallimberti L, Grall-Bronnec M, Herremans SC, Herrmann MJ, Jamil A, Khedr E, Kouimtsidis C, Kozak K, Krupitsky E, Lamm C, Lechner WV, Madeo G, Malmir N, Martinotti G, McDonald W, Montemitro C, Nakamura-Palacios EM, Nasehi M, Noël X, Nosratabadi M, Paulus M, Pettorruso M, Pradhan B, Praharaj SK, Rafferty H, Sahlem G, Salmeron BJ, Sauvaget A, Schluter RS, Sergiou C, Shahbabaie A, Sheffer C, Spagnolo PA, Steele VR, Yuan T-F, van Dongen J, Van Waes V, Venkatasubramanian G, VerdejoGarcía A, Verveer I, Welsh J, Wesley MJ, Witkiewitz K, Yavari F, Zarrindast M-R, Zawertailo L, Zhang X, Cha Y-H, George TP, Frohlich F, Goudriaan AE, Fecteau S, Daughters SB, Stein EA, Fregni F, Nitsche MA, Zangen A, Bikson M, Hanlon CA (2019). Neuroscience & Biobehavioral Reviews. 2019. 104: 118-140
Abstract: There is growing interest in non-invasive brain stimulation (NIBS) as a novel treatment option for substance-use disorders (SUDs). Recent momentum stems from a foundation of preclinical neuroscience demonstrating links between neural circuits and drug consuming behavior, as well as recent FDA-approval of NIBS treatments for mental health disorders that share overlapping pathology with SUDs. As with any emerging field, enthusiasm must be tempered by reason; lessons learned from the past should be prudently applied to future therapies. Here, an international ensemble of experts provides an overview of the state of transcranial-electrical (tES) and transcranial-magnetic (TMS) stimulation applied in SUDs. This consensus paper provides a systematic literature review on published data – emphasizing the heterogeneity of methods and outcome measures while suggesting strategies to help bridge knowledge gaps. The goal of this effort is to provide the community with guidelines for best practices in tES/TMS SUD research. We hope this will accelerate the speed at which the community translates basic neuroscience into advanced neuromodulation tools for clinical practice in addiction medicine.
NYC Neuromodulation Online 2020 Conference will be a virtual, self-organized and self-managed meeting spanning 3 days. The conference will run continuously from April 20th at 9 AM (ET) to April 22nd 9 PM (ET). Any member of the scientific community can develop and submit a session and registration is free of charge and open to everyone.
All meetings must be conducted via Zoom video-conferencing software. After an organizer submits a session, it goes into the review phase by our Conference Scientific Committee. When the session is approved it is posted on our website and participants can start reserving their seats.
NYC Neuromodulation started as a grass-roots non-profit meeting first held in 2015 at the City College of New York in New York City. In 2017, NYC Neuromodulation was run again at The City College of New York. In 2018, NYC Neuromodulation was run jointly with the North American Neuromodulation Society (NANS) at the Sheraton, New York City, In 2019, NYC Neuromodulation was run jointly with the Neuromodulation: The Science conference in Napa, California. The NYC Neuromodulation Online 2020 conference continues in grass-roots spirit, opening up the conference attendance and organization to the neuromodulation community.
New publication:
Front. Hum. Neurosci., 18 March 2020 | https://doi.org/10.3389/fnhum.2020.00077
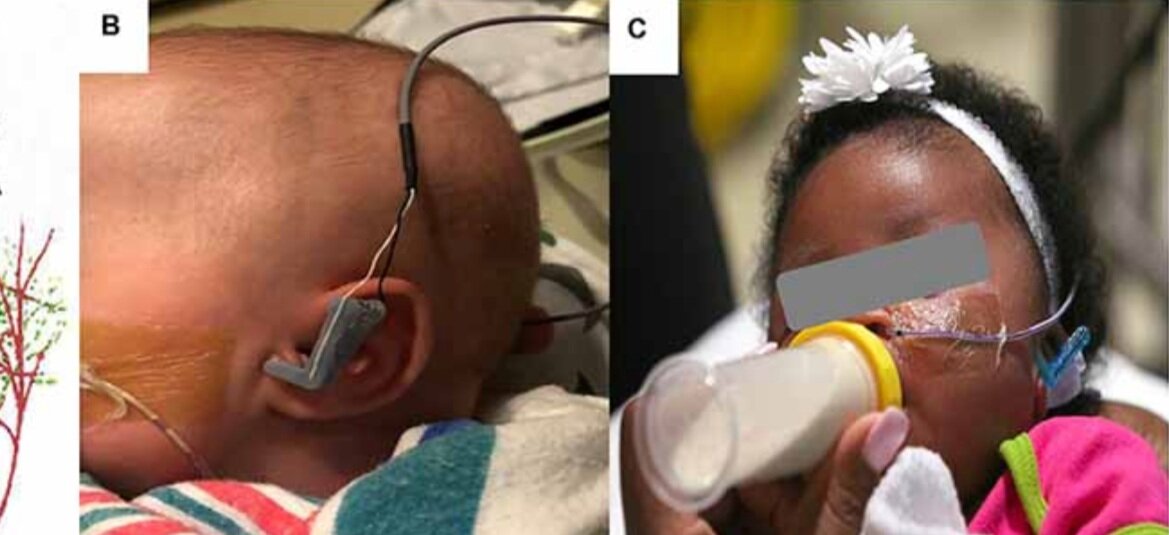
Transcutaneous Auricular Vagus Nerve Stimulation-Paired Rehabilitation for Oromotor Feeding Problems in Newborns: An Open-Label Pilot Study
Bashar W. Badran, Dorothea D. Jenkins, Daniel Cook, Sean Thompson, Morgan Dancy, William H. DeVries, Georgia Mappin, Philipp Summers, Marom Bikson and Mark S. George
Abstract: Neonates born premature or who suffer brain injury at birth often have oral feeding dysfunction and do not meet oral intake requirements needed for discharge. Low oral intake volumes result in extended stays in the hospital (>2 months) and can lead to surgical implant and explant of a gastrostomy tube (G-tube). Prior work suggests pairing vagus nerve stimulation (VNS) with motor activity accelerates functional improvements after stroke, and transcutaneous auricular VNS (taVNS) has emerged as promising noninvasive form of VNS. Pairing taVNS with bottle-feeding rehabilitation may improve oromotor coordination and lead to improved oral intake volumes, ultimately avoiding the need for G-tube placement. We investigated whether taVNS paired with oromotor rehabilitation is tolerable and safe and facilitates motor learning in infants who have failed oral feeding. We enrolled 14 infants [11 premature and 3 hypoxic–ischemic encephalopathy (HIE)] who were slated for G-tube placement in a prospective, open-label study of taVNS-paired rehabilitation to increase feeding volumes. Once-daily taVNS was delivered to the left tragus during bottle feeding for 2 weeks, with optional extension. The primary outcome was attainment of oral feeding volumes and weight gain adequate for discharge without G-tube while also monitoring discomfort and heart rate (HR) as safety outcomes. We observed no adverse events related to stimulation, and stimulation-induced HR reductions were transient and safe and likely confirmed vagal engagement. Eight of 14 participants (57%) achieved adequate feeding volumes for discharge without G-tube (mean treatment length: 16 ± 6 days). We observed significant increases in feeding volume trajectories in responders compared with pre-stimulation (p < 0.05). taVNS-paired feeding rehabilitation appears safe and may improve oral feeding in infants with oromotor dyscoordination, increasing the rate of discharge without G-tube, warranting larger controlled trials.