New paper: tDCS in hyperacute stroke
Matej Slovak, David Kemlink, Pavel Dusek, Petra Rekova, Vratislav Fabian, Martin Jurka, Davide Carone, Alistair Perry, George W.J. Harston, Evzen Ruzicka, Dagmar Altmanova, Lukas Lambert, Andrea Burgetova, Helena Knotkova, Abhishek Datta, Marom Bikson, Michael A. Nitsche, Mersedeh Bahr-Hosseini, Jeffrey L. Saver, Transcranial direct current stimulation is safe and feasible in hyperacute ischemic stroke (DICAST-SF trial), Neurotherapeutics, 2026, https://doi.org/10.1016/j.neurot.2026.e00844
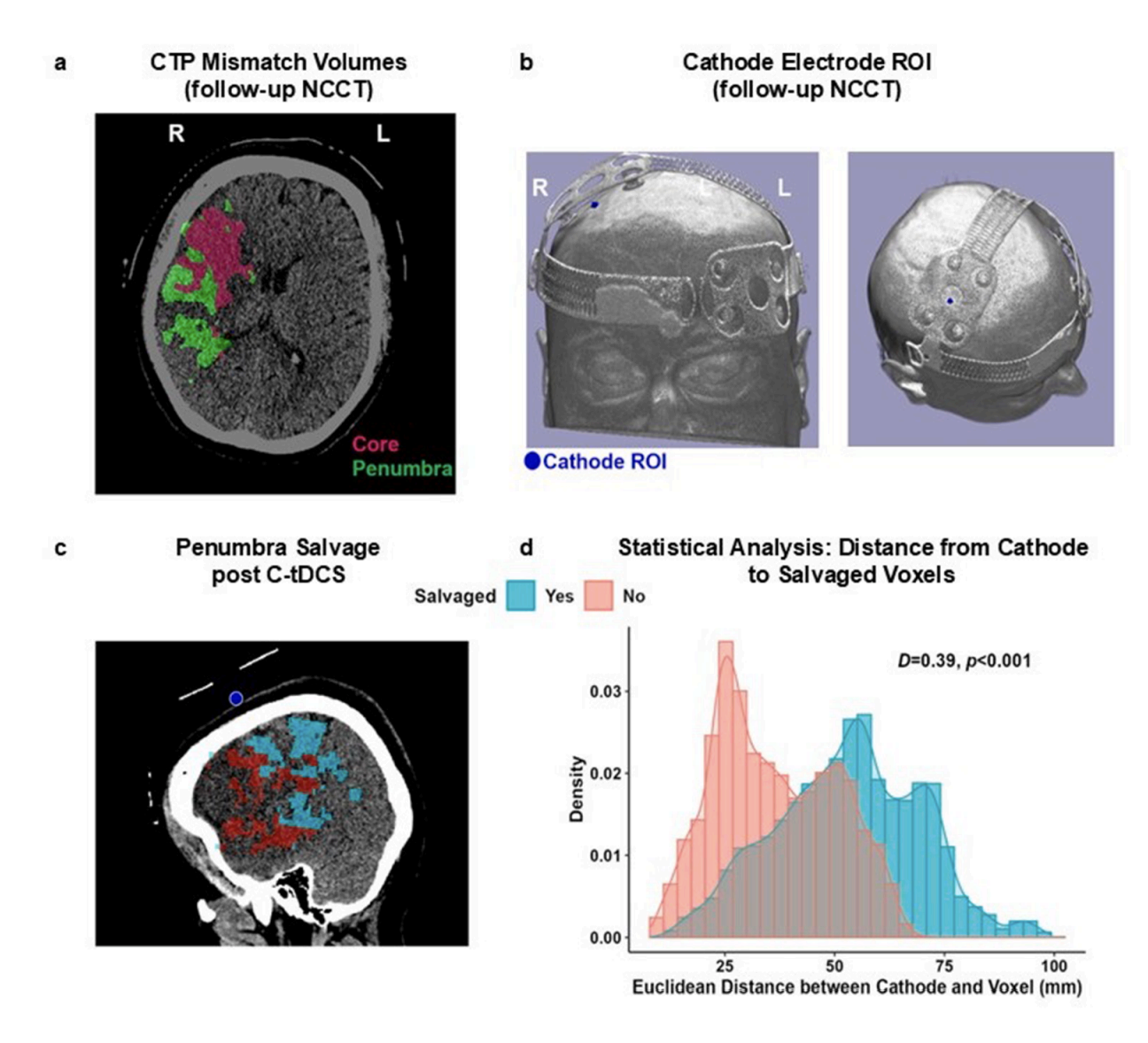
Abstract: Cathodal transcranial direct current stimulation (C-tDCS) is a potential neuroprotective method in the hyperacute phase of ischemic stroke. We aimed to assess safety, tolerability, feasibility, and potential efficacy of C-tDCS in stroke patients with salvageable penumbra. DICAST-SF was a double-blind, randomized, sham-controlled (3 active: 1 sham), 3 + 3 dose-escalation trial. Inclusion criteria were stroke due to occlusion of the internal carotid or middle cerebral artery, last known well time within 24 h, substantial penumbra on CT perfusion, and ineligibility for mechanical thrombectomy. We applied C-tDCS at six dose tiers over the affected primary motor cortex. The primary safety outcome was the symptomatic intracranial hemorrhage (SICH) rate at 24 h post-stimulation. Secondary outcomes included the rates of asymptomatic intracranial hemorrhage (AICH), early neurological deterioration, serious adverse events, and 90-day mortality. Tolerability was assessed by completion rate and questionnaires. Feasibility threshold was defined as median randomization-to-C-tDCS start time within 10 min in the last ten patients. Twenty five patients were enrolled (19 active, 6 sham), mean age 81 (SD 12) years, 16 women, median NIHSS 8 (IQR 6–16). Ten active and 4 sham patients were treated with thrombolysis. No SICH occurred. Three AICH (2 post-thrombolysis) occurred in the active arm. Rates of early deterioration, serious adverse events, and mortality (4 active vs. 2 sham) were comparable. C-tDCS was well tolerated and feasible, median randomization-to-C-tDCS start time was 8 (7–9) min. C-tDCS in hyperacute stroke was safe, well tolerated, and feasible. Findings support further evaluation in larger efficacy trials.
Trial registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04801446.