Marom Bikson lectues at NJIT on April 19. 2024 on “Design of non-invasive electrical brain stimulation”
Slides PDF
Marom Bikson lectues at NJIT on April 19. 2024 on “Design of non-invasive electrical brain stimulation”
Slides PDF

Marom Bikson speaks at the ANT Neuromeeting April 10-11, 2024 in Philadelphia, PA.
Dr. Bikson speaks on Biomarkers for Design. Slides PDF

In Neuromodulation Technology and the Neural Interface. “A Brief History of Slow Spinal Potentials, Gate Theory of Pain, and Spinal Cord Stimulation”
Marom Bikson PhD, Mahima Sharma PhD

Antônio Felipe Lopes Cavalcante, Joanna Sacha Cunha Brito Holanda, João Octávio Sales Passos, Joyce Maria Pereira de Oliveira, Edgard Morya, Alexandre H. Okano, Marom Bikson, Rodrigo Pegado,
Anodal tDCS over the motor cortex improves pain but not physical function in chronic chikungunya arthritis: Randomized controlled trial.
Annals of Physical and Rehabilitation Medicine,, Volume 67, Issue 4, 2024, 101826, ISSN 1877-0657,
https://doi.org/10.1016/j.rehab.2024.101826.

The Bikson lab will be at the 2024 North American Neuromodulation Society (NANS) meeting on Jan 18-21, 2024.
Dr. Bikson is co-chairing the pre-meeting course “Engineering Principles of Deep Brain and Spinal Cord Stimulation” Jan 17, 8 AM- 5 PM. Dr. Bikson will give a lecture in the course on “Subthreshold Mechanisms of Brain and Spinal Cord Stimulation” at 10:20. Lecture slides: PDF
Mohamad Fallahrad will speak on “Highly Deployable and Wearable Non-invasive Electrical Stimulation” on Jan 20, 11:18 AM.
G. Soleimani, J. Joutsa, K. Moussawi, S.H. Siddiqi, R. Kuplicki, M. Bikson, M.P. Paulus, M.D. Fox, C.A. Hanlon, H. Ekhtiari. (2023) Converging Evidence for Frontopolar Cortex as a Target for Neuromodulation in Addiction Treatment. American Journal of Psychiatry. https://doi.org/10.1176/appi.ajp.20221022
Oct 18, 2023, Prof. Marom Bikson gives (via zoom) the Louisiana State University at Baton Rouge psychiatry grand rounds on ““A simple introduction to how neuromodulation devices work.”
Download slides PDF

Prof. Marom Bikson explains, with minimal technical language, How Neuromodulation For Pain Works.
Download SLIDES pdf
The lecture answers: What is "dose" in neuromodulation? How do neuromodulation devices control dose / what are "dose instructions"? When therapies work, what is it that is proven to work? What is the role of mechanisms in the invention of neuromodulation therapies?
What is the special role of the Gate Control Theory of Pain (by Melzack and Wall) and how did it drive modern neuromodulation for pain?
What is Transcutaneous Electrical Nerve Stimulation (TENS)? What is Peripheral Nerve Stimulation (PNS)? What is Spinal Cord Stimulation (SCS)? Dorsal Root Ganglion Stimulation (DRGs)? What are Evoked Compound Action Potentials (eCAPS)? What are Evoked Synaptic Activity Potentials (eSAPS)?
The lecture uses pain as the example but the broader concepts apply to all forms of neuromodulation / brain-stimulation.
Links to the cited works.
1. Fundamentals of Transcranial Electric and Magnetic Stimulation Dose: Definition, Selection, and Reporting Practices: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3346863/
2. Pain Mechanisms : A New Theory https://www.science.org/doi/10.1126/science.150.3699.971
3. Novel Evoked Synaptic Activity Potentials (ESAPs) Elicited by Spinal Cord Stimulation: https://www.eneuro.org/content/10/5/ENEURO.0429-22.2023.long
Citation: Bremner JD, Gazi AH, Lambert TP, Nawar A, Harrison AB, et al. Noninvasive Vagal Nerve Stimulation for Opioid Use Disorder. Ann Depress Anxiety. 2023; 10(1): 1117. PDF
Noninvasive Vagal Nerve Stimulation for Opioid Use Disorder
J Douglas Bremner, MD1,2,3; Asim H Gazi, BS4; Tamara P Lambert, MPH, MEng5; Afra Nawar, BS4; Anna B Harrison, MS4; Justine W Welsh, MD1; Viola Vaccarino, MD, PhD6,7; Kevin M Walton, PhD8; Nora Jaquemet, BS1; Kellen Mermin-Bunnell, BS1; Hewitt Mesfin, BS1; Trinity A Gray, BS1; Keyatta Ross, BS1; Georgia Saks BS5; Nikolina Tomic, MS4; Danner Affadzi, BS1; Marom Bikson, PhD9; Amit J Shah, MD, MSCR3,6,7; Kelly E Dunn, PhD10; Nicholas A Giordano, PhD, RN11; Omer T Inan, PhD4,5
1 Department of Psychiatry & Behavioral Sciences, Emory University School of Medicine, Atlanta GA
2 Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta GA
3 Atlanta Veterans Affairs Healthcare System, Decatur GA 4School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA
5 Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA
6 Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA
7 Department of Medicine, Division of Cardiology, Emory University School of Medicine, Atlanta GA
8 Clinical Research Grants Branch, Division of Therapeu- tics and Medical Consequences, National Institute on Drug Abuse, Bethesda, MD
9 Department of Biomedical Engineering, The City College of New York, New York, NY
10 Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore MD
11 Nell Hodgson Woodruff School of Nursing, Emory Uni- versity, Atlanta, GA
Abstract
Background: Opioid Use Disorder (OUD) is an escalating public health problem with over 100,000 drug overdose-related deaths last year most of them related to opioid overdose, yet treatment options remain limited. Non-invasive Vagal Nerve Stimulation (nVNS) can be delivered via the ear or the neck and is a non-medi- cation alternative to treatment of opioid withdrawal and OUD with potentially widespread applications.
Methods: This paper reviews the neurobiology of opioid with- drawal and OUD and the emerging literature of nVNS for the ap- plication of OUD. Literature databases for Pubmed, Psychinfo, and Medline were queried for these topics for 1982-present.
Results: Opioid withdrawal in the context of OUD is associated with activation of peripheral sympathetic and inflammatory sys- tems as well as alterations in central brain regions including ante- rior cingulate, basal ganglia, and amygdala. NVNS has the potential to reduce sympathetic and inflammatory activation and counter the effects of opioid withdrawal in initial pilot studies. Preliminary studies show that it is potentially effective at acting through sym- pathetic pathways to reduce the effects of opioid withdrawal, in addition to reducing pain and distress.
Conclusions: NVNS shows promise as a non-medication ap- proach to OUD, both in terms of its known effect on neurobiology as well as pilot data showing a reduction in withdrawal symptoms as well as physiological manifestations of opioid withdrawal.

Giuseppina Pilloni, Hyein Cho, Tian Esme Tian, Joerg Beringer, Marom Bikson, Leigh Charvet. (2023) . Immediate and Differential Response to Emotional Stimuli Associated With Transcranial Direct Current Stimulation for Depression: A Visual-Search Task Pilot Study.
Neuromodulation. https://doi.org/10.1016/j.neurom.2023.07.006 in press

Carine El Jamal, Ashley Harrie, Annalise Rahman-Filipiak, Alexandru D Iordan, Alexandre F DaSilva, Robert Ploutz-Snyder, Lara Khadr, Michael Vesia, Marom Bikson, Benjamin M Hampstead. (2023)
Tolerability and blinding of high-definition transcranial direct current stimulation among older adults at intensities of up to 4 mA per electrode.
Brain Stimulation. https://doi.org/10.1016/j.brs.2023.08.025 in press
Marom Bikson organizes the Neuromodulation The Science (NTS) component of the 30th Napa Pain Conference, in Napa, California, on August 18-19, 2023. Program and details.
Dr. Bikson will lecture on “How Neuromodulation for Pain Works.” . Download talk slides

Dr. Marom Bikson lectures on “Neuro-vascular modulation: what a new mechanism suggests about how brain stimulation works and how to interpret hemodynamic imaging?” at the 3rd Annual Brain & Human Body Modeling (BHBM) Conference
(Online format with in-person participation) August 17-18, 2023
Slides PDF

Soleimani, G., Nitsche, M. A., Bergmann, T. O., Towhidkhah, F., Violante, I. R., Lorenz, R., Kuplicki, R., Tsuchiyagaito, A., Mulyana, B., Mayeli, A., Ghobadi-Azbari, P., Mosayebi-Samani, M., Zilverstand, A., Paulus, M. P., Bikson, M., & Ekhtiari, H. (2023). Closing the loop between brain and electrical stimulation: towards precision neuromodulation treatments. Translational Psychiatry, 13, 279(2023). https://doi.org/10.1038/s41398-023-02565-5 PDF

PAPER
Niranjan Khadka, Cynthia Poon, Limary M Cancel, John M Tarbell and Marom Bikson
Published 24 July 2023
Journal of Neural Engineering, Volume 20, Number 4 Citation Niranjan Khadka et al 2023 J. Neural Eng. 20 046014DOI 10.1088/1741-2552/ace4f4
Paper PDF
Objective. Transcranial direct current stimulation (tDCS) generates sustained electric fields in the brain, that may be amplified when crossing capillary walls (across blood-brain barrier, BBB). Electric fields across the BBB may generate fluid flow by electroosmosis. We consider that tDCS may thus enhance interstitial fluid flow. Approach. We developed a modeling pipeline novel in both (1) spanning the mm (head), μm (capillary network), and then nm (down to BBB tight junction (TJ)) scales; and (2) coupling electric current flow to fluid current flow across these scales. Electroosmotic coupling was parametrized based on prior measures of fluid flow across isolated BBB layers. Electric field amplification across the BBB in a realistic capillary network was converted to volumetric fluid exchange. Main results. The ultrastructure of the BBB results in peak electric fields (per mA of applied current) of 32–63 across capillary wall and >1150 in TJs (contrasted with 0.3 in parenchyma). Based on an electroosmotic coupling of 1.0 × 10−9 – 5.6 × 10−10 per , peak water fluxes across the BBB are 2.44 × 10−10 – 6.94 × 10−10, with a peak 1.5 × 10−4 – 5.6 × 10−4 interstitial water exchange (per mA). Significance. Using this pipeline, the fluid exchange rate per each brain voxel can be predicted for any tDCS dose (electrode montage, current) or anatomy. Under experimentally constrained tissue properties, we predicted tDCS produces a fluid exchange rate comparable to endogenous flow, so doubling fluid exchange with further local flow rate hot spots ('jets'). The validation and implication of such tDCS brain 'flushing' is important to establish.

Clinical Trial JAMA Network Open. 2023 Jun 1;6(6):e2319231. doi: 10.1001/jamanetworkopen.2023.19231.
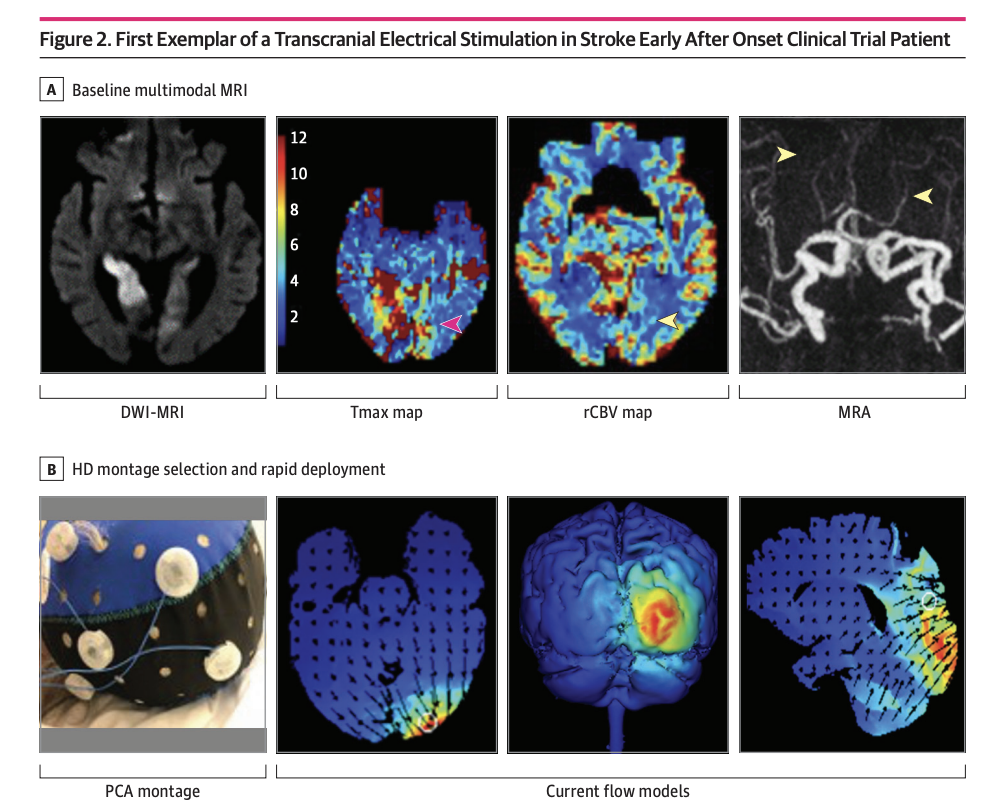
High-definition Cathodal Direct Current Stimulation for Treatment of Acute Ischemic Stroke: A Randomized Clinical Trial
Mersedeh Bahr-Hosseini, Kambiz Nael, Gozde Unal, Marco Iacoboni, David S Liebeskind, Marom Bikson, Jeffrey L Saver; TESSERACT Trial Group
PMID: 37342040 PMCID: PMC10285579 DOI: 10.1001/jamanetworkopen.2023.19231
Key Points
Question Is application of high-definition cathodal transcranial direct current stimulation (HD C-tDCS) as a noninvasive targeted acute ischemic stroke treatment strategy feasible and well-tolerated, and does it show signals of beneficial effects?
Findings In this randomized clinical trial enrolling 10 patients (7 active, 3 sham), HD C-tDCS was started within a median 12.5 minutes of randomization in final enrolled patients and showed good tolerability with signals of favorable effects on salvage of threatened tissue.
Meaning These results suggest that HD C-tDCS is a noninvasive targeted acute ischemic stroke treatment strategy that can be efficiently applied in emergency settings and warrants testing in larger multicenter trials.
Abstract
Importance Cathodal transcranial direct current stimulation (C-tDCS) provides neuroprotection in preclinical models of acute ischemic stroke (AIS) by inhibiting peri-infarct excitotoxic effects and enhancing collateral perfusion due to its vasodilatory properties.
Objective To report the first-in-human pilot study using individualized high-definition (HD) C-tDCS as a treatment of AIS.
Design, Setting, and Participants This randomized clinical trial was sham controlled with 3 + 3 dose escalation design, and was conducted at a single center from October 2018 to July 2021. Eligible participants were treated for AIS within 24 hours from onset, had imaging evidence of cortical ischemia with salvageable penumbra, and were ineligible for reperfusion therapies. HD C-tDCS electrode montage was selected for each patient to deliver the electric current to the ischemic region only. Patients were followed for 90 days.
Main Outcomes and Measures Primary outcomes were feasibility, assessed as time from randomization to study stimulation initiation; tolerability, assessed by rate of patients completing the full study stimulation period; and safety, assessed by rates of symptomatic intracranial hemorrhage at 24 hours. The efficacy imaging biomarkers of neuroprotection and collateral enhancement were explored.
Results A total of 10 patients with AIS were enrolled, 7 were randomized to active treatment and 3 to sham. Patient age was mean (SD) 75 (10) years old, 6 (60%) were female, and National Institutes of Health Stroke Scale score was mean (SD) 8 (7). Two doses of HD C-tDCS (1 milliamp [mA] for 20 minutes and 2 mA for 20 minutes) were studied. The speed of HD C-tDCS implementation was a median (IQR) 12.5 minutes (9-15 minutes) in the last 4 patients. Patients tolerated the HD C-tDCS with no permanent stimulation cessation. The hypoperfused region was reduced by a median (IQR) 100% (46% to 100%) in the active group vs increased by 325% (112% to 412%) in sham. Change in quantitative relative cerebral blood volume early poststimulation was a median (IQR) 64% (40% to 110%) in active vs −4% (−7% to 1%) sham patients and followed a dose-response pattern. Penumbral salvage in the active C-tDCS group was median (IQR) 66% (29% to 80.5%) vs 0% (IQR 0% to 0%) in sham.
Conclusion and Relevance In this randomized, first-in-human clinical trial, HD C-tDCS was started efficiently and well tolerated in emergency settings, with signals of beneficial effect upon penumbral salvage. These results support advancing HD C-tDCS to larger trials.
Trial Registration ClinicalTrials.gov Identifier: NCT03574038
Nigel Gebodh, PhD Candidate mentored by Professor Marom Bikson has recently been highlighted by the NIH-funded Graduate Research Training Initiative for Student Enhancement (G-RISE) Program. Learn more about Nigel and his research by watching his video below!